10 years building colorectal cancer collection in Luxembourg: an additional tool for translational research
22 April 2021
The collection of tumour samples from various Luxembourg hospitals has enabled to launch several research projects, including European and international ones, which already present very promising results.
Initiated in 2011 by the University of Luxembourg and the Integrated Biobank of Luxembourg (IBBL) as a concerted action against colorectal cancer (CRC), the collection of tumour samples from various Luxembourg hospitals has enabled to launch several research projects which already present very promising results for the treatment of colorectal cancer patients.
Prof. Serge Haan and Dr. Elisabeth Letellier from the Department of Life Sciences and Medicine (DLSM) at the University of Luxembourg who launched the project in 2011 explain in more details the importance of such a collection.

How did the collection start?
In 2010, we gathered with Dr. Jos Even from the Laboratoire National de Santé (LNS) and the scientific management team from the IBBL to investigate colorectal cancer by setting up a high-quality tissue collection from colon cancer patients in Luxembourg. IBBL, in the context of its mission of serving the Luxembourg research community, decided to make this collection one of its strategic initiatives. The project started with the support of the Fondation Cancer and the Luxembourg National Research Fund (FNR).
Over the years, the collection has grown significantly with the launch of several research projects and the support of many partners such as the Laboratoire National de Santé (LNS), the Centre d’Investigation et d’Épidémiologie Clinique (CIEC/LIH), the Centre Hospitalier Emile Mayrisch (CHEM).
What is the current status of the collection?
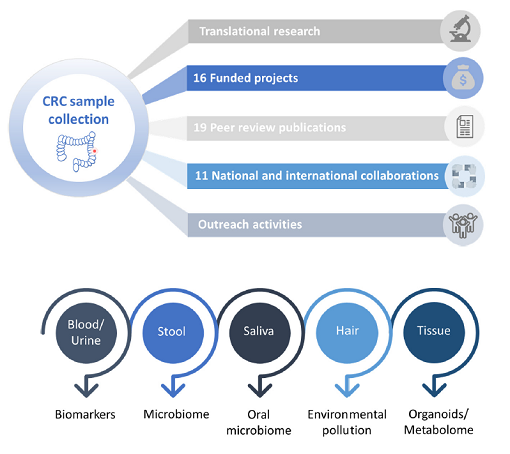
Over the past years, we have established an ongoing collection of tumour tissue samples from CRC patients, assembling high quality samples of over 170 patients. This collection contains a multitude of sample types, such as serum, plasma, immune cells, stool and tumour tissue and normal counterparts from the same patients. Clinical parameters are available for all samples (age, gender, tumour location, survival, diet surveys, therapies, etc.), allowing for studies, which can generate highly valuable translational findings. Pre-analytical factors, such as the cold and warm ischemic time, the Bristol score, as well as dietary questionnaires are collected along with the samples.
We have a follow-up for these patients every year up to 5 years and clinical data is collected for more than 10 years. As the progression of CRC takes over 10 years, samples and data (survival, treatment etc.) covering 10 years are required to have a clinical relevant collection. This is why establishing such a cohort is a future-oriented project which will yield a lot of important translational findings in the domain of gastrointestinal cancers over the next years.
What is its value?
The collection allows generating results that can be translated into a clinical setting. Importantly, this cohort has a unique added value, based on (i) complete sets of sample types, (ii) full preanalytical documentation and characterisation, (iii) longitudinal follow-up samples, (iv) extended clinical data annotations, (v) quality control measurements. As we collect the tumour cells as well as the different cells of the tumour microenvironment, we can generate “small tumours” in the lab which nicely recapitulate the original tumour. These models allow us to study the mechanisms underlying tumour initiation as well as progression but also the development of novel drugs that not only target the tumour cells but also its microenvironment. This is crucial as the past years have clearly demonstrated that the tumour microenvironment plays a key role in tumour progression. Building up these complex tumour models from a patient’s material allows to develop drugs that are specific for this patient. In essence, it fosters personalised medicine.
Over the past years, we have acquired extensive knowledge in the exploitation of the results generated with the samples of the cohort. For example, by using our CRC cohort, we have identified promising biomarkers with a strong prognostic value in early CRC stages. One of our recently identified biomarkers has led to the filing of a patent on novel biomarkers for cancer diagnosis, prediction, or staging. Together with the IBBL, we obtained a Proof of Concept funding from the FNR to test one of the identified biomarkers for clinical use.
What is the future of the collection?
We have already initiated in collaboration with different groups at the University but also the Luxembourg Institute of Health (LIH) and international partners several research projects which are using the samples from the cohort or the cultures derived from them. As an example, IBBL was invited to join a European project, partly based on the value of our collection. These tools are important to generate high translational results. In addition, we have established a biobank at the University which contains 3D spheroid cultures, organoids, as well as cells of the tumour microenvironment such as fibroblasts. This biobank can be used by researchers to perform mechanistic studies as well as drug profiling or biomarker studies.
We would like to expand this cohort and involve more hospitals as for example the Hôpitaux Robert Schuman, Centre Hospitalier de Luxembourg and Centre Hospitalier du Nord. Our future aim is to include this cohort into the National Cancer Plan and further develop it as a national cohort that can be used by all researchers in Luxembourg and abroad.








